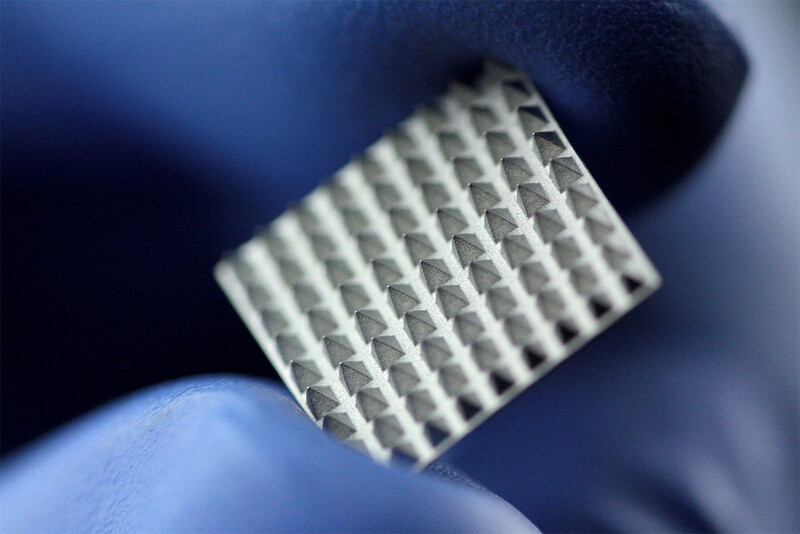
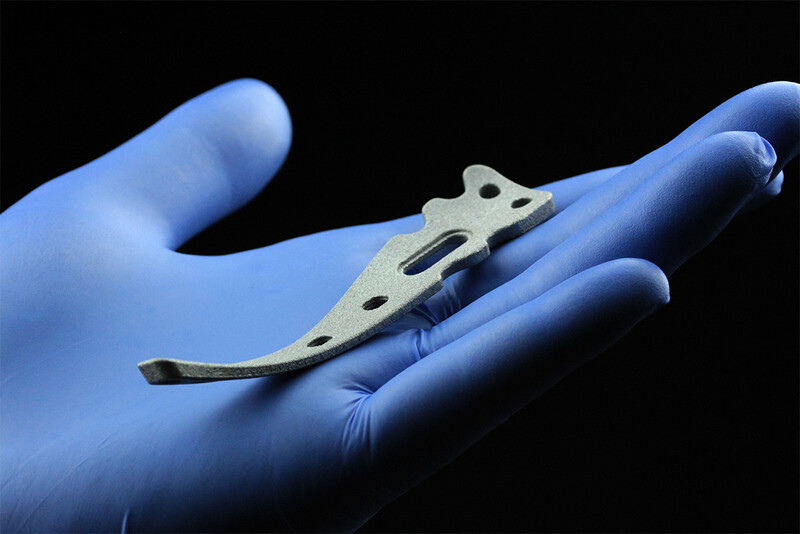
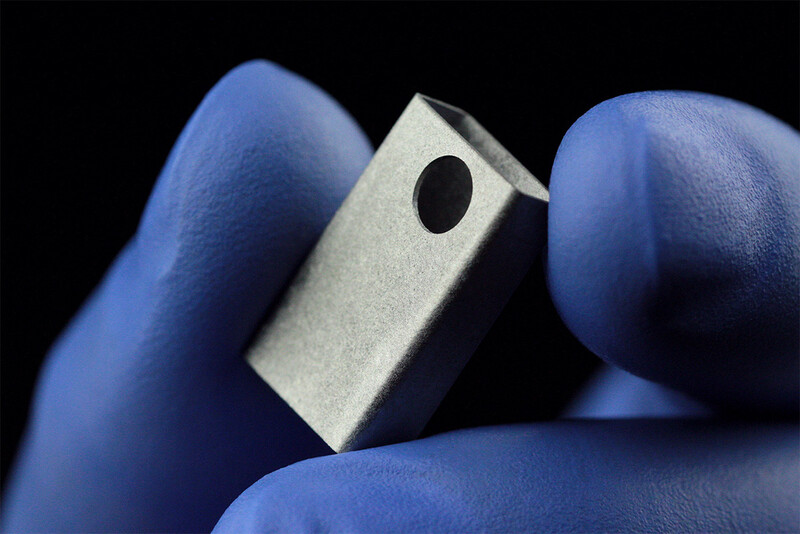
Medical
Element22 manufactures parts and components for medical applications in the fields of Cardiac Rhythm Management, Orthopedics, Spine, Dental and many more.
Our customers in the medical industry are located around the globe. The components we manufacture have been tested in depth for many different properties like chemistry, static and dynamic performance, density, stress corrosion cracking, biocompatibility, toxicity, weldability, and more, with excellent results.
Part Examples
- CRM: Feedthrough flanges, Housings, Terminal connector blocks & screws
- Cervical plates & screws
- Polyaxial Pedicle screws: body, tulip, load ring
- Vertebral spacers
- Artificial cervical & lumbar discs
- Femoral Head / Femoral Ball / Acetabular Shell
- Wrist plates & screws, ankle plates & screws, elbow plates & screws
- Dental implants, mini implants, abutments, angled abutments, abutment screws, braces -brackets
- Phacoemulsification tips, glaucoma stents
- And many more…
Quality & Certification
Element22 is qualified according to ISO 9001 and ISO 13485. The Titanium MIM components we manufacture have FDA approval since 2012.
We offer different Grades of Titanium. The materials follow international standards such as:
- ASTM F 2885 - Standard Specification for Metal Injection Molded Titanium-6Aluminum-4Vanadium Components for Surgical Implant Applications
- ASTM F 2989 - Standard Specification for Metal Injection Molded Unalloyed Titanium Components for Surgical Implant Applications